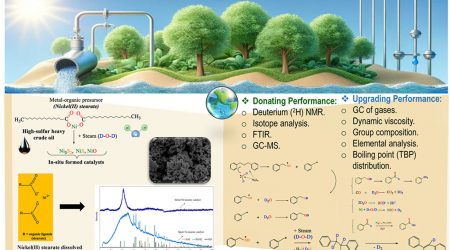
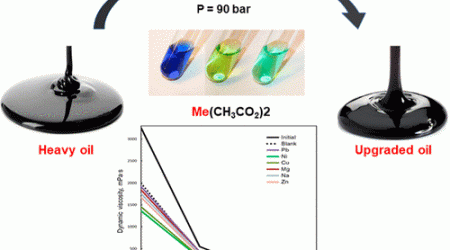
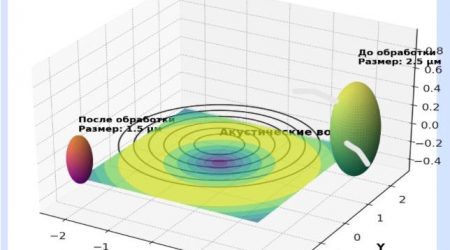
KFU working on various hydrate formation promoters
Scientists have synthesized a new surfactant containing sulfonate, amide and carboxyl groups (SSAC). The research was conducted at the Laboratory of Hydrate Technologies of Utilization and Storage of Greenhouse Gases with the support of the Ministry of Science and Higher Education of Russia.
Today, one of the most promising methods of natural gas storage and transportation is to turn it into gas hydrate. This solid crystalline substance looks like ice. One volume of hydrate can hold up to 170 volumes of gas, which clearly confirms the prospects of this method. It is an alternative to liquefied natural gas and pipeline transportation technologies. At the same time, “solid gas” can be applied under milder conditions than LNG. This technology is especially interesting for regions with more extreme climatic conditions, where it is very difficult and expensive to build pipelines. Another advantage is that it is the safest of all gas storage and transportation technologies.
In a recent study, scientists at Kazan University managed to significantly accelerate the formation of gas hydrates. The SSAC promoter was synthesized by combining the chemical properties of the two most effective groups of gas pedals amino acids and sulfated surfactants (e.g., sodium dodecyl sulfate (SDS)), which fundamentally distinguishes it from existing reagents. Unlike known analogs, the new promoter does not form a large amount of foam during hydrate decomposition. The reagent has successfully passed laboratory tests.
“A highly effective hydrate formation promoter can either create milder reaction conditions or accelerate the rate of hydrate formation. In our study on kinetic promoters of hydrate formation, we observed that these types of promoters enhance hydrate formation kinetics by promoting interactions between gas and water molecules. The efficiency of promoters is evaluated not only by increasing the rate and reducing the time of hydrate formation, but also by such factors as gas consumption, storage capacity at low concentrations and the ability to release gas back without foam formation,” noted Design Engineer Sadeh Elaheh.
“The creation of each new compound often involves a lot of trial and error, but we were fortunate to achieve truly unique results,” emphasized the researcher.
The priority of this research work is to develop a kinetic promoter of hydrate formation that should outperform known commercial promoters such as sodium dodecyl sulfate (SDS). The next stage of the research will be to take the development to commercial scale.
“The synthesized promoter is the first to meet all the necessary criteria: relatively simple synthesis, excellent kinetic characteristics, foam-free dissociation process and, most importantly, high efficiency on gas-to-hydrate conversion at very low concentrations, which significantly reduces the cost of chemical reagents. Now we plan to work out in detail the technological use of the new promoter for the conditions of certain fields,” said Laboratory Head Mikhail Varfolomeev.